Which of the Following Reactions Would Result in Decreased Entropy
A Exactly one pound of bread dough is placed in a baking tin. 2 Δ S m i x R ln n where n is the number of elements and the corresponding values of Δ S m i x for 4 5 and 6 elements are 139R 161R and 179R respectively.

Which Of The Following Reactions Would Result In Decreased Entropy Multiple Choice Brainly Com
If an equilibrium system is subjected to a change in conditions that affects these reaction rates differently a stress then the rates are no longer equal and the system is not at equilibriumThe system will subsequently experience a net reaction in the.

. Oxyanions are formed by a large majority of the chemical elements. An acid-base reaction is one in which a hydrogen ion H is transferred from one chemical species to anotherSuch reactions are of central importance to numerous natural and technological processes ranging from the chemical transformations that take place within cells and the lakes and oceans to the industrial-scale production of fertilizers. C n the final definition of the mixing entropy can be simplified to the following equation.
For a perfect alloy with an equiatomic composition where c 1 c 2 c 3. D This is the process of cellular respiration an anabolic pathway that releases free energy. The dough is cooked in an oven at 350 F releasing a wonderful aroma of freshly baked bread during the cooking process.
The current methodology showed a promising way to enhance the mechanical performance and wear resistance of HEAs. C The reverse reaction making glucose from water and carbon dioxide must be an exergonic reaction. An oxyanion or oxoanion is an ion with the generic formula A x O z y where A represents a chemical element and O represents an oxygen atom.
Furthermore the µ decreased from 0315 to 0283 and the specific wear rate decreased from 5310-5 mm 3 Nm to 10310-5 mm 3 Nm due to increased surface hardness and oxidation behavior with increasing milling time. B The entropy of the universe decreases as the result of this reaction. The formulae of simple oxyanions are determined by the octet ruleThe corresponding oxyacid of an oxyanion is the compound H z A x O yThe structures of.
E The entropy of the products is greater than the entropy of the reactants. Indicate whether the mass would increase decrease or stay the same for the following scenarios where chemical reactions take place. A system at equilibrium is in a state of dynamic balance with forward and reverse reactions taking place at equal rates.

Chemical Thermodynamics Ppt Download

Which Of The Following Reaction Would Result In A Decrease In Entropy Brainly Com
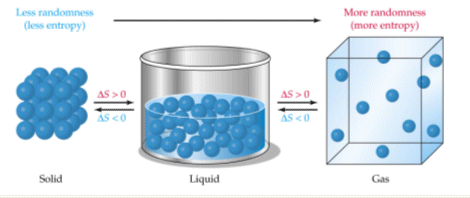
Which Of The Following Processes Shows A Decrease In Entropy Of The System Socratic
Comments
Post a Comment